The Fetal Cell Count™ Kit is used for the diagnosis of fetomaternal hemorrhage (FMH). It is a complete kit for routine diagnosis of fetomaternal hemorrhage using anti-carbonic anhydrase (CA) and anti-fetal hemoglobin (HbF).
Key benefits
- Unique quantification of fetomaternal hemorrhage by flow cytometry
- Complete assay for routine diagnosis of fetomaternal hemorrhage
- Simple and easy handling procedure
- FETALtrol™ available as system control (not validated for use with the Fetal Cell Count™ Kit).
Features
- Discriminates between fetal cells and maternal HbF+ cells
- Detection as low as 0.014% fetal cells in maternal blood
- Based on the patented combination of antibodies against fetal hemoglobin and carbonic anhydrase
- Results within 90 minutes
- Registered as Medical Device for In Vitro Diagnostic Use (IVD/CE)
Applications
- Determination of fetomaternal hemorrhage
- Pregnancy with suspected RhD incompatibilities
- Abdominal trauma
Introduction
Detection and quantification of fetal red blood cells (fRBCs) in maternal blood samples is essential for obstetrical management.
Measurement of fRBCs is critical as the extent of fetomaternal hemorrhage (FMH), the transplacental passage of fRBCs into the maternal circulation, has consequences for further treatment of mother and child. Frequency and size of FMH is directly influenced by complications in abdominal trauma, suspected placental injury or after a caesarean section.
Severe FMH may lead to intra-uterine death. In case of antigen incompatibility between mother and child FMH may result in respiratory problems or anemia in newborns.
Adult RBC’s contains a population of HbF+ cells (F-cells), which can differ in size between 0 and 14%. The F-cells can give a positive result in the Kleihauer-Betke acid-elution test or single HbF flow cytometry test. These F-cells may be the result of physiological variations during pregnancy or traits of thalassemia, sickle cell anaemia or hereditary persistence of fetal haemoglobin.
The detection (and enumeration) of fRBCs is used to calculate the extent of FMH, either in case of trauma with suspected placental injury or in a situation of RhD incompatibility between the fetus and the mother. The amount of fRBCs is a measure to determine the correct dose for the (prophylactic) anti-D therapy in order to prevent hemolytic disease in the newborn.
Principle of the Fetal Cell Count™ Kit
The Fetal Cell Count™ Kit is a flow cytometry assay. The assay is based on a patented combination of two antibodies. One is directed against HbF while the second is specific for carbonic anhydrase (CA), an enzyme present in adult RBCs and, at very low detectable level, in late pregnancy stage in fetal RBCs.
• Precise and accurate detection of fRBCs
• Intracellular detection of HbF and CA
• Distinguishes between fetal RBCs, maternal F-cells and maternal RBCs
• Eliminates subjective interpretations an empiric signal cutoffs
• Results within 90 minutes
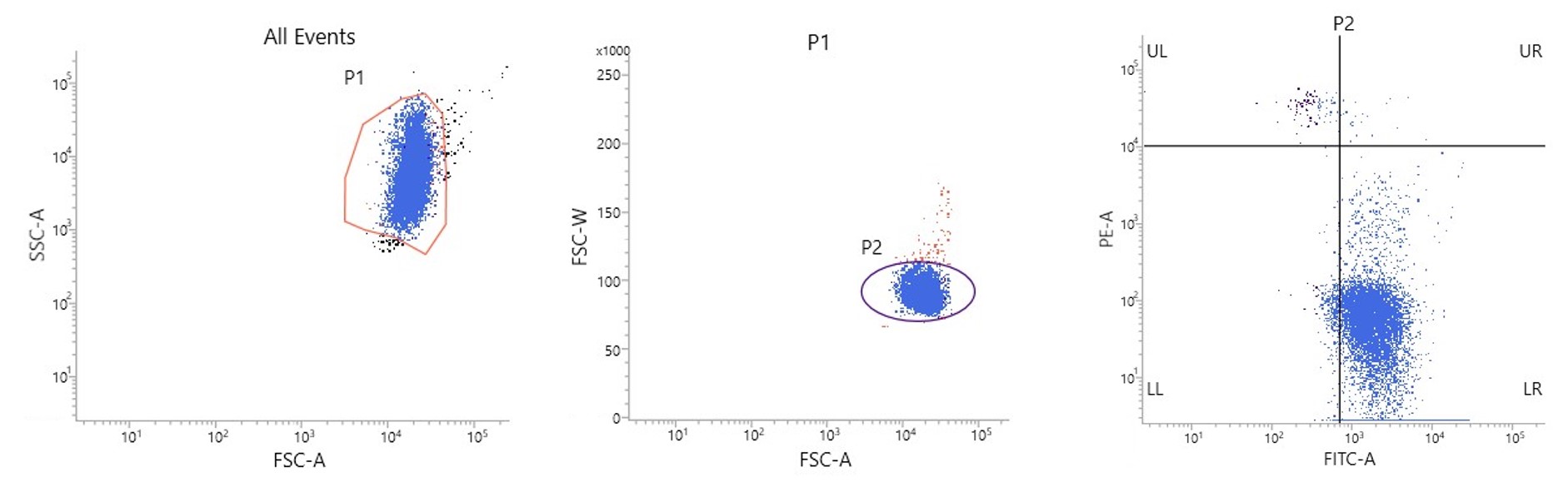 Figure 1. Dotplots illustrating typical data obtained with the Fetal Cell Count™ kit: A) FSC/SSC plot to identify the red blood cell population. B) FSC-A/FSC-W plot to exclude doublets. C) CA-FITC/HbF plot to identify fetal red blood cells (UL).
Figure 1. Dotplots illustrating typical data obtained with the Fetal Cell Count™ kit: A) FSC/SSC plot to identify the red blood cell population. B) FSC-A/FSC-W plot to exclude doublets. C) CA-FITC/HbF plot to identify fetal red blood cells (UL).
Conclusion
The data demonstrate the usefulness of CA as a red blood cell marker. It allows, in combination with HbF, an accurate discrimination between the different RBC populations in maternal blood. Without the use of this second marker, discrimination between the fetal RBCs and the variable concentrations of HbF containing maternal F-cells becomes less precise.
1. Valérie Porra et al, Identification and quantification of fetal red blood cells in maternal blood by a dual-color flow cytometric method:evaluation of the Fetal Cell Count kit, Transfusion, 2007;47:1281-1289
2. Liesbeth Bakker-Jonges et al, The Detection of Foetal Red Blood Cells in Maternal Blood Samples by Dual Flow Cytometry, Haematology, 2008, 58-60
3. Marek Lubusky et al, Fetomaternal hemorrhage in normal vaginal delivery and in delivery by cesarean section, Transfusion, 2012
4. Waltraut M Merz et al, Dual-colour flow cytometry for the analysis of Fetomaternal haemorrhage during delivery, J Clin Pathol, 2012;65;No 2:186-187
Question: I use cord blood as a positive control but I detect CA+HBF+ cells in my sample. Should I include these cells in my Hbf+ cell gate?
Answer: No, even though these CA+Hbf+ cells are likely to be present in cord blood. Already very late in pregnancy, fetal red blood cells start to express CA to prepare for birth and life outside the womb. However, analysis has learned that the number of events in Q2 in general is negligible compared to the HbF+ cell population in Q1. In addition, when a patient sample is analyzed Q2 may contain maternal F-cells. Therefore, this quadrant is excluded when considering Hbf+ fetal erythrocytes.
When analyzing the data, it is essential to always place the vertical line F directly left from the maternal F-cells (CA+/HbF+) and the horizontal line just below the fetal Hbf+ cell population, as shown in figure 1. All cells in the upper region in Q4 are maternal erythrocytes (F cells) that express both CA and Hbf (F cells) and should not be included in Q2.
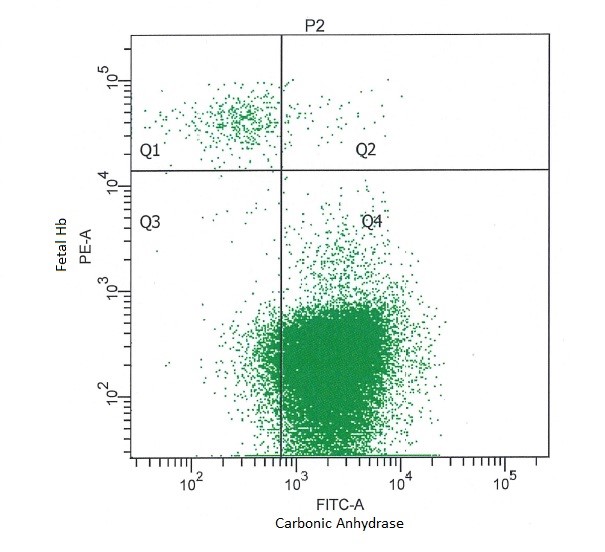
Figure 1. Representative result obtained with a sample containing 1% umbilical cord and 99% whole blood (male) analyzed with the Fetal Cell Count kit.
Question: What is the clinically relevant percentage of Hbf+ cells to state FMH?
Answer: The threshold to determine whether these is a clinically relevant amount of FMH is 0.1% Hbf+ cells1. The calculated amount of mL can be used to either calculate the dose of anti-D or, in case of a trauma, to assess the impact of the bleeding with knowledge of the age of the fetus and its (estimated) blood volume.
Question: How long can a blood sample be stored before performing the assay and in what conditions?
Answer: Blood samples should be stored at either 2-8 °C or at room temperature (20–25 °C) up to 3 days until processing.
Question: Can you use blood from newborns instead of cord blood as a positive control for HbF?
Answer: Yes that is possible, but the HbF signal can be lower and the CA signal will be higher than cord blood fetal cells. This will result in a lower percentage cell detected when mixed with an adult blood sample.
Question: What is recommended to use as positive control for HbF if you don’t have cord blood available?
Answer: FETALtrol™ can be used as an external control (not validated for use with the Fetal Cell Count™ Kit).
1 Guidelines for the Estimation of Fetomaternal Hemorrhage. E Austin, S Abtes, M de Silva, D Howarth, A Lubenko, M Rowley, M Scott, E Thomas, J White, M Williams. (January 2011).