Control cells to validate the procedures for the detection of Fetomaternal Hemorrhage
Key benefits
- Eliminates the need to acquire cord blood to make home brew controls, saving time precious to the laboratory workforce
- Ideal control when fresh cord blood is not available
Features
- Three validated levels which correspond to clinical decision points for anti-D therapy
- 3 months total shelf life
- FDA cleared as a hematologic control for fetal red cell detection
- Registered as Medical Device for In Vitro Diagnostic Use (IVD/CE)
Applications
- Flow cytometric methods
- Kleihauer-Betke tests
- EQA programs
Order now!
Order your products via our order form!
Product code: FH101 (3 levels, two 2 mL vials
of each level)
Product code: FH102 (3 levels, one 2 mL vial of each level)
Introduction
Detection and quantification of fetal red blood cells (RBCs) in maternal blood samples is essential for obstetrical management. Measurement of fetal RBCs is critical as the extent of Fetomaternal Hemorrhage (FMH), the transplacental passage of fetal RBCs into the maternal circulation, has consequences for further treatment of mother and child.
Frequency and size of FMH is directly influenced by complications in abdominal trauma, suspected placental injury or after a caesarean section. Severe FMH may lead to intra-uterine death. In case of antigen incompatibility between mother and child FMH may result in respiratory problems or anemia, like Hemolytic Disease of the Newborn.
Principle of the FETALtrol™
The laboratory determination of the level of fetal cells in maternal circulation remains an important support in the obstetrical management of women with suspected uterine trauma and in the proper dose administration of Rh immune globulin. Limitations in the sensitivity and precision of the widely used manual Kleihauer-Betke method have prompted can increased utilization of flow cytometric methods for fetal cell detection in maternal blood samples.
The anti-HbF flow cytometric method for detection of fetal cells offers a simple, reliable and more precise alternative to the KB manual technique for the assessment of FMH. The method has additional potential applications for the study of HbF levels or frequency of adult red cells with low levels of HbF (F cells) in individuals with hemoglobinopathies.
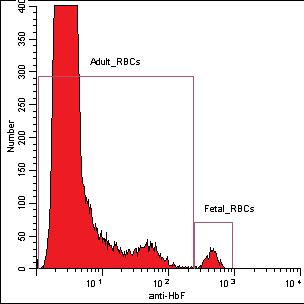
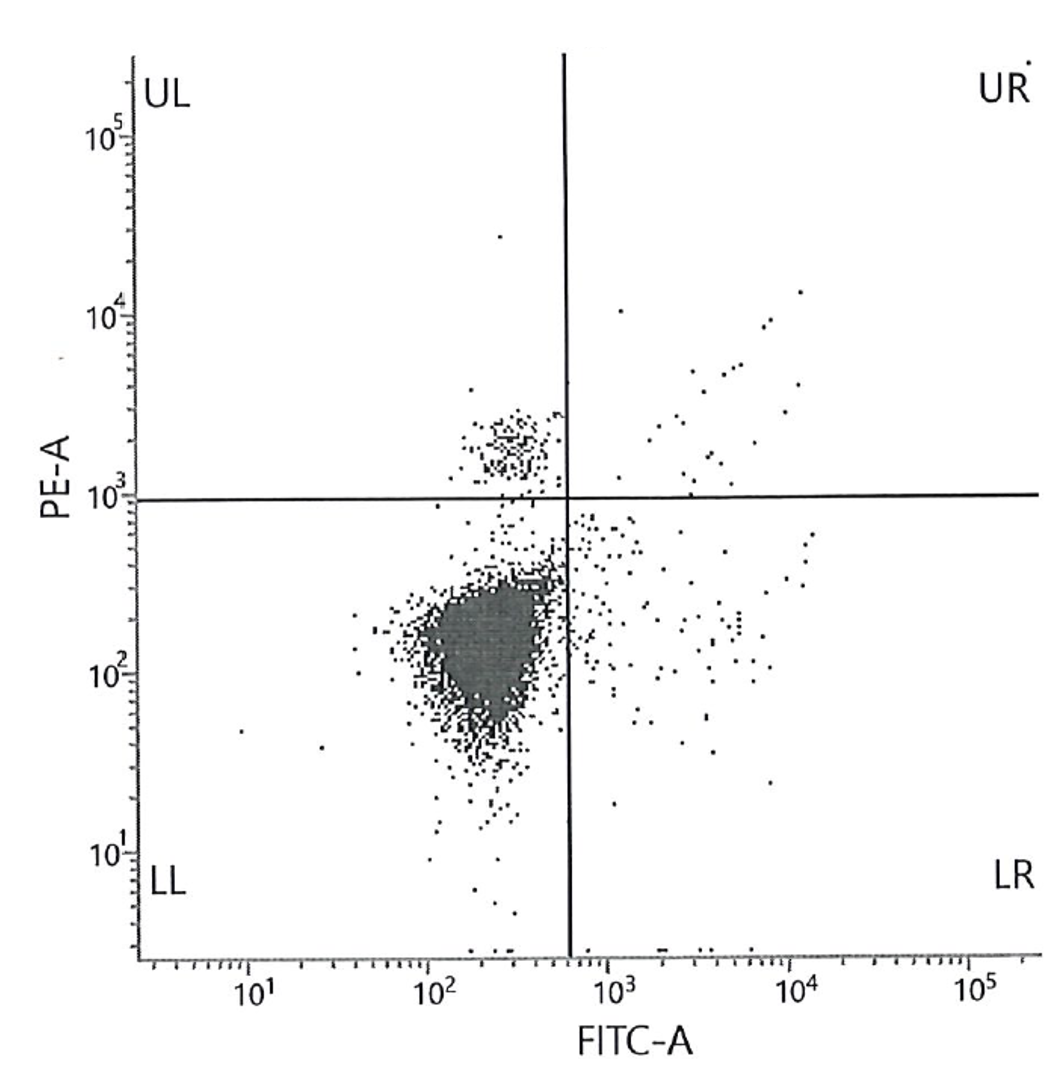
Figure 1. Histogram (A) shows the results of the high level (3) of FETALtrol™ when used in the FMH QuikQuant™. Dotplot (B) shows the results of the high level of FETALtrol™ when used in the Fetal Cell Count™ Kit. Please note that FETALtrol™ is not validated for use with the Fetal Cell Count™ Kit
Question: What is recommended to use as positive control for HbF if you don’t have cord blood available?
Answer: FETALtrol can be used as an extern control. This is a quality controlled tri-level stabilized blood control with known HbF positive cells content in adult blood for the FMH QuikQuant™. Please note that FETALtrol™ is not validated for the use in the Fetal Cell Count™ Kit
Please contact us to receive the instructions for use and the latest assay sheet with expected ranges.