Collagen hybridizing peptide
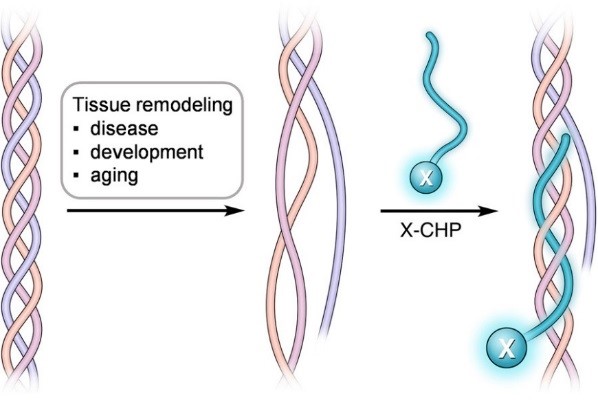
Collagen has a unique triple helical structure that is unfolded in tissues during diseases, development, mechanical injury, and decellularization. The collagen hybridizing peptide (CHP) is a novel and unique peptide that specifically binds unfolded collagen chains, both in vitro and in vivo1,2,3, enabling a straightforward detection of inflammation and tissue damage caused by a large variety of diseases, as well as tissue remodeling during development and aging1.
Figure 1. By sharing the structural and sequence motif of natural collagen, CHP has a strong capability to hybridize with denatured collagen strands, in a fashion that is similar to a DNA fragment annealing to its complimentary DNA strand during PCR1,4.
CHP is available with conjugates for fluorescence detection: 5-Carboxyfluorescein (F-CHP) or sulfo-Cyanine3 (R-CHP) or for avidin/streptavidin-mediated detection a biotin conjugation (B-CHP) (Table 1).
Table 1. Available CHP conjugates.
| Item | Description | Regulatory status | Package size | Product Code |
| Collagen Hybridizing Peptide, 5 FAM Conjugate (F-CHP)1 | CHP coupled with 5-FAM for immunofluorescence | RUO | 60 μg | FLU60 |
| 300 μg | FLU300 | |||
| Collagen Hybridizing Peptide 5-FAM Conjugate, Scrambled Control1 | CHP 5-FAM scrambled control | RUO | 60 μgs | FLU60CTL |
| Collagen Hybridizing Peptide, Cy3 Conjugate (R-CHP)1 | CHP coupled with Cy3 for immunofluorescence | RUO | 60 μg | RED60 |
| 300 μg | RED300 | |||
| Collagen Hybridizing Peptide Cy3 Conjugate, Scrambled Control1 | CHP Cy3 scrambled control | RUO | 60 μgs | RED60CTL |
| Collagen Hybridizing Peptide, Biotin Conjugate (B-CHP)1 | CHP coupled with biotin for immunohistochemistry and SDS-PAGE | RUO | 60 μg | BIO60 |
| 300 μg | BIO300 | |||
| Collagen Hybridizing Peptide Biotin Conjugate, Scrambled Control1 | CHP Biotin scrambled control | RUO | 60 μgs | Bio60CTL |
| 1 Distributed for 3Helix Inc., Salt Lake City, USA | ||||
Features
- More informative, reliable and convenient than zymography, DQ collagen, SHG, and TEM
- High affinity and unparalleled specificity to collagen with essentially no nonspecific binding
- Applicable to all types of collagen from all species, relying on collagen’s secondary structure instead of any defined sequence for binding
- Suitable for both frozen and paraffin-embedded sections with no need for antigen retrieval
- A non-antibody approach with no species restrictions against any co-staining antibody
- Small size (2% of IgG by MW) enabling facile tissue penetration and whole specimen staining without sectioning
- Stable in solution under 4 °C, eliminating the need to aliquot for storage
Applications
- Immunofluorescence
- Immunohistochemistry
- Cell Imaging
- SDS-PAGE (in-gel Western blot)
Order now!
Order your products via our order form!
Product codes: FLU60, FLU300, BIO60, BIO300, RED60 and RED300
Introduction
By sharing the Gly-X-Y repeating sequence of natural collagen, CHP has a strong capability to hybridize with denatured collagen chains by reforming the triple helical structure, in a fashion similar to DNA fragments annealing to complementary DNA strands1,2,4. CHP is extremely specific: it has negligible affinity to intact collagen molecules due to lack of binding sites, and it is inert towards non-specific binding because of its neutral and hydrophilic nature.
CHP is a powerful histopathology tool which enables straightforward detection of inflammation and tissue damage caused by a large variety of diseases, as well as tissue remodeling during development and aging1. CHP robustly visualizes the pericellular matrix turnover caused by proteolytic migration of cancer cells within 3D collagen culture, without the use of synthetic fluorogenic matrices or genetically modified cells3. CHP can measure and localize mechanical injury to collagenous tissue at the molecular level5. It also enables assessment of collagen denaturation in decellularized extracellular matrix6. In addition, CHP can be used to specifically visualize collagen bands in SDS-PAGE gels without the need for western blot7.
- Targeting collagen strands by photo-triggered triple-helix hybridization. Proceedings of the National Academy of Sciences of the United States of America, 2012, 109, 14767–14772. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3443117/
- In situ imaging of tissue remodeling with collagen hybridizing peptides. ACS Nano, 2017, 11, 9825–9835. https://www.ncbi.nlm.nih.gov/pubmed/28877431
- Visualizing collagen proteolysis by peptide hybridization: From 3D cell culture to in vivo imaging. Biomaterials, 2018, 183, 67–76. https://www.sciencedirect.com/science/article/pii/S0142961218305933
- Targeting and mimicking collagens via triple helical peptide assemblies. Current Opinion in Chemical Biology, 2013, 17, 968– 975. https://www.ncbi.nlm.nih.gov/pubmed/24210894
- Molecular level detection and localization of mechanical damage in collagen enabled by collagen hybridizing peptides. Nature Communications, 2017, 8, 14913, doi: 10.1038/ncomms14913. https://www.nature.com/articles/ncomms14913
- Molecular assessment of collagen denaturation in decellularized tissues using a collagen hybridizing peptide. Acta Biomaterialia, 2017, 53, 268–278. https://www.sciencedirect.com/science/article/pii/S1742706117300892
- Direct detection of collagenous proteins by fluorescently labeled collagen mimetic peptides. Bioconjugate Chemistry, 2013, 24, 9–16. https://pubs.acs.org/doi/abs/10.1021/bc3005842?journalCode=bcches
For a full list of references click here
Question: How stable are the CHP products in solution at 4 °C? Can the CHP reagents be aliquoted and stored at -20 °C or -80 °C after reconstitution? Why it is recommended to store the powder at -20 °C before reconstitution?
Answer: The peptide is highly stable in solution during storage. Figure 1 shows the stability profiles of the two reagents stored in an aqueous solution at 4 °C. As can be seen, the purity barely decreases following one year storage in solution. However, if the user wishes, all CHP products can be aliquoted and stored at -20 °C or -80 °C. In fact, there may not be a need to aliquot. The peptides are stable and do not denature or degrade after multiple freeze-thaw cycles.
We recommend -20 °C as the storage conditions of the powder just to ensure the products have the highest quality when they are used in the first experiment. We understand that the products are sometimes stored for months upon arrival before they are opened.
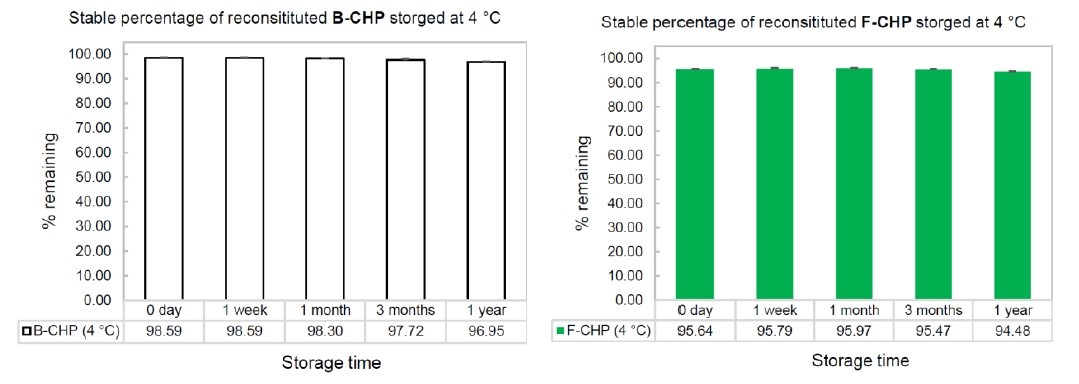
Figure 1. Stability profiles of B-CHP and F-CHP when stored in aqueous solution at 4 ˚C up to 1 year.
Question: This peptide has only one label molecule in one peptide molecule. Is this correct? If so, do you control the length of the peptide in production? If the CHP products are a mixture of GXY peptides of different lengths, accurate calculation of molar in one vial would be difficult.
Answer: Correct, the CHP probes have only one labeling moiety in one peptide molecule. The peptides are chemically synthesized on solid phase. They are not made by recombinant methods. As such, we accurately control the exact length of the peptides in production. All CHP molecules in a unit vial and in all batches are exactly the same (e.g., same length, same molecular weight). We verify every batch of our products closely with HPLC and MALDI MS.
Question: Does the probe need to be heated up prior to use each time or only when reconstituting? If the un-dilute stock solution is exposed to multiple heat-cool dissociation procedure, will the CHP still be stable?
Answer: Yes. CHP needs to be heated up prior to use EACH TIME. Once it is cooled down and stored at 4 °C for a while in solution, it will gradually re-assemble into the trimeric form.
After years of working with CHP, we found no limit of its heating/cooling cycles. The peptide is very stable both chemically and physically. However, we do NOT recommend users to heat up the stock solution. It is not necessary. The recommended procedure is that a user only aliquot out the needed amount from the stock solution (e.g., 10 μL, 50 μM), and dilute it to the required concentration (e.g., 50 μL, 10 μM) with PBS. Subsequently, the stock solution should be returned to 4 °C storage, and ONLY the diluted solution is heated and active for the experiment. In this way, the stock solution is not going to be heated and cooled down for multiple cycles. Only the diluted solution will be heated once.
Question: In step 5 for staining protocol (B), if the dead time for dispensing is over 3 min, will something wrong happen?
Answer: If the dead-time for dispensing is over 3 min, the experiment will still work well. The heat-dissociated CHP strands take hours to re-assemble. A short delay of a few minutes is not going to cause a problem. But please do not wait for hours to use after heating. We recommend including a normal control tissue slide in a histology study, so that the pathological slide of interest are stained together with the normal control with the same dead time. The two will be directly comparable.
However, please note that the single strand CHP still re-assembles after heating (at a very slow speed in the diluted solution), so to achieve maximal binding, we still recommend users to minimize the cooling and dispensing dead time.
Question: Why do you perform incubation at 4 °C (e.g., step 6 in protocol B)? If this step is performed at room temperature (RT) or 37 °C, what will happen?
Answer: We recommend performing staining incubation at 4 °C for these reasons:
(1) CHP’s affinity to denatured collagen is stronger at 4 °C than at RT and 37 °C. The user can perform binding at RT or 37 °C, but colder temperature will achieve a higher level of binding.
(2) CHP is often used in immunostaining of frozen tissue slides. These unfixed tissue sections need to be maintained at 4 °C during staining.
Question: The step 6 in protocol B mentions optimal staining with overnight incubation, but 2 h minimum. How different are the stains after 2 h incubation vs overnight?
Answer: The answer to this question is published in ref 2,
Link: https://pubs.acs.org/doi/suppl/10.1021/acsnano.7b03150/suppl_file/nn7b03150_si_001.pdf
Question: What kind of fixative or fixation technique is recommended? Will the staining result depend on fixation?
Answer: As described in detail in ref 2, the staining result does not depend on fixation in our tests. Fixation is not necessary for CHP staining. However, if fixing is required for other reasons, the tissue can be chemically fixed before CHP staining. For fluorescent imaging (e.g., when using F-CHP or R-CHP), we recommend using formaldehyde, instead of paraformaldehyde or glutaraldehyde, to fix the tissue, because the formaldehyde fixed tissues have much less auto-fluorescence, especially in the FITC fluorescence channel. If the user has to use paraformaldehyde or glutaraldehyde, to overcome the possible autofluorescence issue, we recommend staining the tissue with R-CHP, or B-CHP and detecting the B-CHP binding with a streptavidin conjugate that is labeled with a red / near-infrared dye (e.g., AlexaFluor647) or is labeled with an enzyme (e.g. HRP for non-fluorescence detection).
Question: How to co-stain a slide with CHP and an antibody? If a slide is pre-stained by immunohistochemistry, can it be stained again by CHP?
Answer:
A tissue slide can be readily co-stained with CHP and an antibody together. The primary antibody can be directly diluted into the CHP solution after the CHP solution is heated and cooled down to room temperature. As such, the slides are co-stained with CHP and the antibody together overnight at 4 °C. The antibody can be detected by labeled 2nd antibody following incubation. The protocol describing the co-staining steps are briefly mentioned in protocol (B) and (C). Detailed examples and protocols are reported in ref [2] and [3].
We have not tested whether the CHP will still work or not if the samples have been pre-stained by immunohistochemistry. It may depend on what the exact immunohistochemistry is and what detection method is used to visualize CHP binding.
If conditions allow, we recommend using un-stained slides for CHP imaging analysis to get the most reliable results.
Question: If antigen retrieval is necessary for immunohistochemistry, may these treatment influence CHP staining?
Answer: Our tests indicate that the CHP staining is drastically enhanced after the tissue is treated by the heat-mediated antigen retrieval (AR) process (unpublished). We believe that the AR treatment can denature all the collagen molecules in the tissue, and the CHP staining following AR simply reveals the total collagen content in the tissue. If AR is necessary for immunohistochemistry, we recommend performing CHP staining on a separate, neighboring slide that is not treated with AR. This way, the CHP staining is only detecting the naturally degraded or denatured collagen.
Question: Would it be better to use the biotin-labeled CHP (B-CHP) to enhance amplification and sensitivity?
Answer: In many cases, we found that all F-CHP, R-CHP, and B-CHP work very well. When higher sensitivity is indeed needed, we found that B-CHP, detected by a fluorescently labeled streptavidin (in the red or far red emission region, such as AlexaFluor647, 555 etc.), gives the best result, due to the multiple fluorophores conjugated to each streptavidin molecule. Horseradish peroxidase (HRP) is another way to enhance the signal, but sometimes there could be some background stain.
Question: Can you recommend a negative control for my histology experiment? How do I know the detected signal is truly due to CHP hybridizing with the unfolded collagen?
Answer: You can verify whether the binding is truly due to CHP-collagen hybridization or non-specific binding by using a CHP solution without the pre-heating step. As described in the protocol above, the CHP single strands have a strong propensity to assemble into the trimeric form in solution at 4 °C. Without the pre-heating step, such folded, trimeric CHP probe has no driving force to bind denatured collagen chains; meanwhile, it has the same chemical natural of CHP, making it a great negative control.
If the sample stained with pre-heated CHP following the normal procedure (e.g., protocol B or C) shows positive signal, whereas a matching sample stained with the same CHP solution only without the pre-heating step (negative control) shows no or significantly reduced CHP signal, the detected CHP binding in the positive sample can be considered truly due to CHP-collagen hybridization and not due to non-specific binding.
To ensure CHP is fully self-assembled into the trimeric form, make the CHP stock solution with a higher than 50 μM concentration (ideally 100-150 μM), store it at 4 °C for at least 2 days, and directly dilute it in cold PBS buffer without heating before using.
Please note that it is not surprising to detect some background fluorescence in normal tissue. This is either because of CHP binding to the low content of degraded collagen in native tissue (caused by the endogenous collagen remodeling) or auto-fluorescence from the tissue.