Diagnosis of Heparin-Induced Thrombocytopenia (HIT)
Complete standardized high quality kit for the diagnosis of HIT
Key benefits
- Complete kit with ready-to-use components
- Functional assay
- Non-radioactive method
- No-wash method
Features
- Rapid results (<2 hr)
- Standard equipment and methodology
- Patented method
- Registered as Medical Device for In Vitro Diagnostic Use (IVD/CE)
Applications
- Detection of heparin complexes specific antibodies
- Reactivity independent of PF4
- Kit also detects IL-8/heparin and NAP-2/heparin complex antibodies
Introduction
Heparin-induced thrombocytopenia (HIT) is an acute, immune-mediated process that may result in life-threatening thrombosis. HIT is caused by platelet-activating antibodies, which bind to complexes consisting of heparin and platelet factor-4 (PF4) (most of the clinical cases), interleukin-8 (IL-8) or neutrophil-activating peptide 2 (NAP-2). Detection of these antibodies is an important laboratory function in the process of setting the diagnosis for HIT.
The antibodies are initially formed after 5 or more days after initiating heparin therapy. An immune response to heparin may be observed within minutes or hours if the patient has had previous exposure to heparin and antibodies are already circulating.
Setting the diagnosis for HIT is difficult because many patients receiving heparin have co-morbid conditions that pre-dispose them to thrombocytopenia, and therefore it may be difficult to be certain of the diagnosis of HIT. Due to the above mentioned mechanism it is very important to look for the presence and function of the complex specific antibodies.
Diagnostics
For setting the diagnosis of HIT, two different kind of assays have to be used: immunological and functional assays.
Immunological assays (antigenemia) detect antibodies reactive to the heparin platelet-factor 4 (PF4) complexes. But only a fraction of patients positive in these tests develop clinical HIT. On the other hand, pathogenic antibodies may also react with other heparin complexes, such as heparin-interleukin-8, or heparin-neutrophil activating peptide 2. These pathogenic antibodies are not detected by the PF4 immunological assays.
Functional assays reproduce the in-vivo pathophysiology, and highly correlate with the clinical presentation of HIT. The serotonin-release assay (SRA) is a functional assay, with high sensitivity and specificity, but is not a preferred test because of the use of radio isotypes. Another functional assay is the aggregation assay but it has a low sensitivity and takes a long time to perform. The HITAlert™ is a functional assay based on flow cytometry and will be explained in more detail below.
Publications (Garritsen, Solano) show that the specificity of the HITAlert™ Kit is in 100% and 99% agreement in comparison with the SRA and a PF4 immunological assay respectively. The sensitivity of the HITAlert™ Kit compared to the SRA was found to be 81%, and 88% when compared with a PF4 immunological assay.
The results of the HITAlert™ Kit should always be used in conjunction with clinical findings (symptoms and 4T test) and other serological tests.
Principle of the HITAlert™ Kit
In 2012, the American College of Chest Physicians (ACCP) posted updates on recommendations for testing of HIT. Here they state that not all of the anti-PF4 antibodies detected by immune assays are capable of causing clinical HIT; hence, the specificity of these tests is only moderate. The guideline describes various cases in which a functional assay must be conducted to confirm the diagnosis of HIT. The HITAlert™ kit is such a functional assay.
For the HITAlert™ Kit donor platelets (PRP) are used, which are incubated in the presence of patient serum and in the presence or absence of heparin. When pathogenic antibodies are present in the patient serum, the activation of the donor platelets is shown using a platelet activation marker. Combining the activation marker with an antibody to identify the platelets, this reaction can be visualized using flow cytometry.
Results
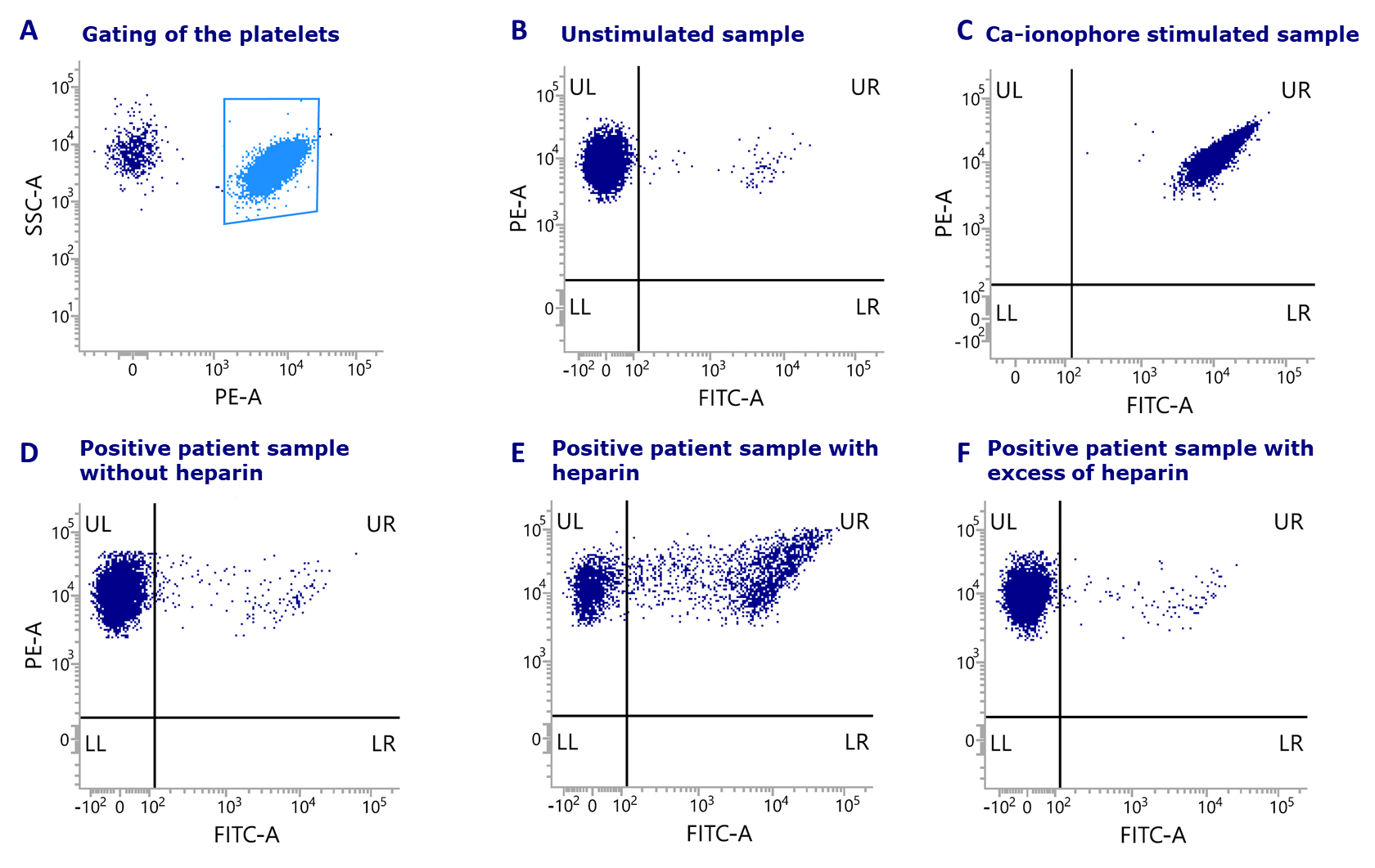
Figure: Results of HITAlert™ Kit shown for each sample including the analysis of a patient sample: (A) Selection of the platelets. (B) Unstimulated sample. (C) Sample stimulated with Ca-ionophore. (D) Positive patient sample without heparin. (E) Positive patients sample with heparin (F) Positive patient sample with excess of heparin.
Garritsen et al., , High sensitivity and specificity of a new functional flow cytometry assay for clinically significant heparin-induced thrombocytopenia antibodies. IJLH 2014; 36(2):135-43.
Solano et al., Using HitAlert flow cytometry to detect heparin-induced thrombocytopenia antibodies in a tertiary care hospital. Blood Coag fibr 2013; 24(4):365-370
Scully et al., Pathologic Antibodies to Platelet Factor 4 after ChAdOx1 nCoV-19 Vaccination. NEJM 2021, April 16
Question: Why can’t you use blood with other additives than citrate (i.e. EDTA, heparin)?
Answer: The platelets cannot be activated when using EDTA anti-coagulated blood, because calcium is depleted by the EDTA. Platelets need calcium for activation.
Usage of heparin anti-coagulated blood will result in a negative test result.
Question: Can you use blood of a donor with another blood type than blood group O?
Answer: No, it is recommended to use O-type blood so there is no interference of the AB antibodies from the serum sample.